ELISA-Entwicklung
GmbH & Co. KG
Range of Services
Assay Development
Assay Validation
Sample Measurement
Protein Purification
Antibody Production
HCP-Assays
Anti Drug Antibody, ADA
Testkits
Recombinant protein pharmaceuticals have to be purified to a high degree from host cell components to avoid adverse drug effects like allergic or anaphylactic reactions. Typically a multianalyte enzyme-linked immunosorbent assay (ELISA) is the method of choice, using polyclonal antibodies to fulfil the following features:
- A simple validated method
- High sensitivity to find trace amounts in a final drug substance
- Exact reproducible quantification
- Recognition of a wide number of epitopes present in the impurities
- Utilisation of Polyclonal antibodies instead of monoclonal
- Usage of a mix of antibodies of different host species (e.g. goat and rabbit),
- Protein A purified antibodies instead of specifically HCP purified antibodies for additional measurement of epitopes with low antigenity,
- Streptavidin coated plates instead of direct antibody coating to reduce antibody denaturation,
- Mix of differently conjugated antibodies to biotin and HRP (antibody linked via lysine and via cysteine),
- Test performance with only one incubation step to reduce antigen loss of low-affinity antibodies
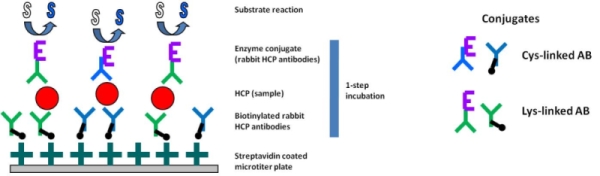
- an excess of streptavidin coating reduces well to well variation and unspecific background
- indirect antibody coating via streptavidin avoids AB binding site denaturation which occurs during direct AB coating
- indirect AB coating increases sensitivity by reducing steric hindrance
- direct AB-enzyme conjugation allows a 1-step incubation assay protocol to reduce dissociation of low affinity AB
- using a combination of Cys- and Lys-linked AB increases antigen binding
- Organization and supervision of antibody production
- Antiserum screening, purification and conjugate syntheses
- 1D and 2D electrophoresis with silver staining for HCP visualization
- Western blotting for demonstration of antibody binding and specifity
- Assay development, validation, documentation and test kit production
- Technology transfer and adaptation at customer lab including training of responsible persons
- Adaptation to automatic systems if desired

