ELISA-Entwicklung
GmbH & Co. KG
Range of Services
Assay Development
Assay Validation
Sample Measurement
Protein Purification
Antibody Production
HCP-Assays
Anti Drug Antibody, ADA
Testkits
back - to samplemeasurement back - to testkits [pdf]

Prinziple of Test Procedure
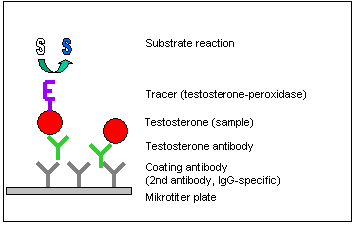
The testosterone enzyme immunoassay is a competitive ELISA in 96-well microtiter plates (stripwell or massive plate format). Stripwell plates (12 x 8-well strips) are the best choice for few samples whereas 96-well plates are much easier to handle for the measurement of hundreds of samples.
In the first incubation step free testosterone (sample, control or standard) competes with Tracer (horseradish peroxidase conjugated testosterone) for a limited number of antigen binding sites of the testosterone specific antibodies. At the same time the antibodies were fixed to the microtiter plates by a secondary antibody bound to the plate surface.
After removing the excess of test compounds by washing, enzyme activity of the tracer is detected with tetramethylbenzidine (TMB) as substrate.
Reagents and Material provided
- Microtiterplate(12 x 8-well strips )
- Testosterone Standards A 0 ng/ml
- Low-Control: 1 ng/ml (0,8 to 1,2 ng/ml) High-Control: 4 ng/ml (3,2 to 4,8 ng/ml)
- 50x Wash Buffer Concentrate Containing 0.01% Thimerosal as a preservative
- 2x Assay Buffer Concentrate BSA in a buffered solution containing dye and 0.01% Thimerosal as a preservative
- Tracer Stock Solution Containing 50% glycerol to prevent freezing. Store at –18°C to –25°C
- Pocine Serum Matrix For Tracer Solution
- Antiserum Stock Solution Containing 50% glycerol to prevent freezing. Store at –18°C to –25°C
- Substrate Solution One-step TMB-Substrate
- Stop Solution 1 M HCl
B 10,0 ng/ml
C 5,0 ng/ml
D 2,5 ng/ml
E 1,25 ng/ml
F 0,63 ng/ml
G 0,31 ng/ml
Warnings and Precautions
- For Research Use only. Not for use in diagnostic procedures.
- Treat samples as potentially biohazardous material. Follow Universal Precautions when handling contents of this kit and any sample.
- Dispose of containers and unused contents in accordance with Federal State and Local regulatory requirements.
- Store assay reagents as indicated.
- Test each sample in duplicate.
- Use of multichannel pipettes or repeat pipettors is recommended to ensure timely delivery of reagents.
- Pipet carefully using only calibrated equipment.
- Perform assay with any validated washing method, preferably, automated plate washer.
- Perform standard curve with each assay.
- Adequate mixing of Standards, Controls, samples and the use of a microtiter plate shaker is required for acceptable assay performance.
Preparation of Reagents
All reagents should be equilibrated to room temperature (20-26°C) prior to use except the Tracer Stock Solution and the Antiserum Stock
Solution.
Be sure that possible precipitates in Buffer Solution Concentrates will be re-dissolved before dilution.
Coated Strips
Remove Stripwell frame and the required number of coated strips from the pouch. Ensure that the pouch containing any unused strips is completely
resealed and contains desiccant.
Wash Buffer
Prepare required amount of Wash Buffer by diluting 50x Wash Buffer Concentrate 1:50 with deionized water. Store at 2-8°C and use Wash Buffer
within 2 weeks of preparation.
E.g. add 10 ml 50x Wash Buffer Concentrate to 490 ml deionized water.
Assay Buffer
Prepare required amount of Assay Buffer by diluting 2x Assay Buffer Concentrate 1:2 with deionized water. Store at 2-8°C and use Assay Buffer
within 2 months of preparation. E.g. add 25 ml Assay Buffer Concentrate to 25 ml deionized water.
Tracer Solution
Avoid warming of the glycerol containing Stock Solutions!
Prepare Tracer solution before use. Do not store.
Glycerolated Stock Solutions are a bit viscous. For pipetting cut off about 3 mm from top of a pipet tip, aspirate the desired volume and dispense
in Assay Buffer. Spill the tip by repeated (5-10 times) aspiration/dispense cycles.
Dilute Testosterone Tracer Stock Solution in Assay Buffer 1:180 and add 10% Porcine Serum Matrix.
Examples:
4 Strips: 1.8 ml Assay Buffer + 11.1 µl Tracer Stock Solution + 200 µl Serum Matrix
12 Strips: 5 ml Assay Buffer + 30.5 µl Tracer Stock Solution + 500 µl Serum Matrix.
Antiserum Solution
Avoid warming of the glycerol containing Stock Solutions!
Prepare Tracer solution before use. Do not store.
Glycerolated Stock Solutions are a bit viscous. For pipetting cut off about 3 mm from top of a pipet tip, aspirate the desired volume and dispense
in Assay Buffer. Spill the tip by repeated (5-10 times) aspiration/dispense cycles.
Dilute Testosterone Antiserum Stock Solution in Assay Buffer 1:500.
Examples:
4 Strips: 2.0 ml Assay Buffer 1x + 4 µl Antiserum Stock Solution
12 Strips: 5.5 ml Assay Buffer 1x + 11 µl Antiserum Stock Solution.
Storage
- Tracer Stock Solution and Antiserum Stock Solution have to be stored at –18°C to –25°C.
- Store all other Kit components at 2-8°C.
Assay Procedure
Read entire product instructions before beginning the assay.
Peroxidase and the TMB Substrate are light sensitive. Avoid direct sunlight.
Cover the plate with aluminium foil during incubation steps.
Sample incubation
1. Allow pouch of Coated Strips/Coated Plate to equilibrate to room temperature before opening. Remove Stripwell Frame and the required
number of Coated Strips from the pouch. Ensure that the pouch containing unused strips is completely resealed and contains desiccant.
2. Place desired number of Coated Strips in Stripwell Frame just prior to use. Label strips to prevent mix-up in case of accidental
removal from Stripwell Frame. Cover the Plate/Strips during all incubation steps.
3. Dilute samples as necessary with Assay Buffer (e.g. 1:50).
4. Pipet 50 µl Tracer Solution to each well by using a multichannel pipette.
5. Add 50 µl Standards, Controls and pre-diluted samples.
6. Add 50 µl Antiserum Solution to each well by using a multichannel pipette.
7. Mix Plate content on a microplate shaker for 5-10 min.
8. Incubate over night (16-24h) at 4°C.
Substrate incubation
9. Wash 4 times:
Empty Strips/Plate. Add at least 250µl of Wash Buffer to each well.
Repeat three more times for a total of four washes.
Preferably, an automated microtiter plate washer should be used.
Vigorously blot the Strips/Plate dry on paper towels after the last wash.
10. Pipet 100 µl of Substrate Solution to each well.
11. Incubate 45 ± 10 min at room temperature.
Stop/Read
12. Add 100 µl of Stop Solution to each well. Add Stop solution in the same pattern and time intervals as the Substrate Solution addition.
13. Read optical density at 450 nm (use 595-650 nm as reference wavelength, if possible). Assure that no large bubbles are present in wells and that the bottom of the Strips/Plate are clean. Strips/Plate should be read immediately (within 5 min).
14. Use appropriate computer software for calibration curve to analyse assay results (e.g. 4-Parameter Method).
15. Determine concentration of samples and Controls from the standard curve. Multiply the values with the dilution factor if pre-dilution of the samples was different to 1:50.
16. Control values should be within the range specified.
The control values are intended to verify the validity of the curve and sample results. Each laboratory should establish its own parameters for acceptable assay limits. If the control values are NOT within this limits, the assay results should be considered as questionable and the samples should be repeated.
Standard concentrations are calculated for serum/plasma samples pre-diluted 1:50 in Assay Buffer.
Example Data and Standard Curve
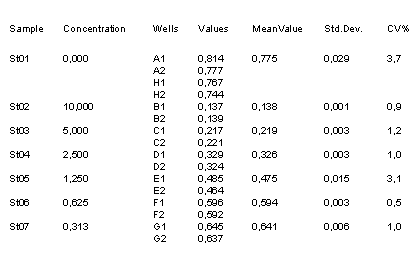
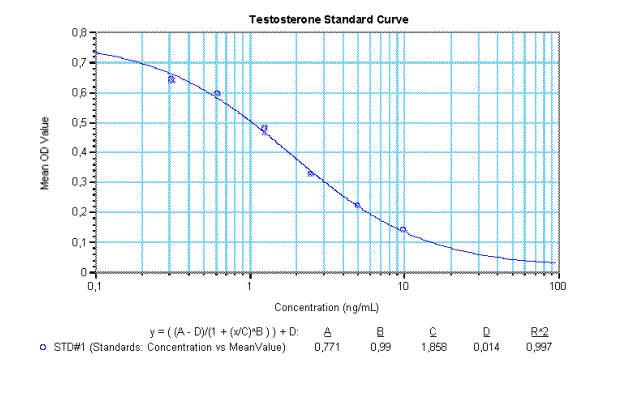
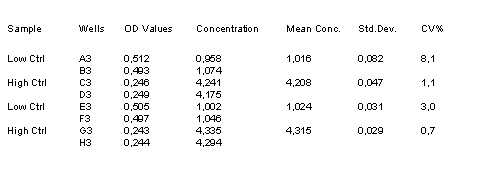
Characteristics of the Testosterone Assay
Samples
Porcine plasma or serum.
Pre-dilution needed for the assay: 1:20 or higher. Standard concentrations are calculated for serum/plasma samples pre-diluted 1:50.
Measuring range
0.3 to 10 ng/ml (50 µl sample: serum pre-diluted 1:50)
Antiserum was raised in rabbits against testosterone-3-CMO-BSA
Finale dilution 1:1,500,000.
Cross-reactivity
Testosterone 100%, 5a-DHT 96%, androstendione 3%, progesterone < 0.01%, 17ß estradiol < 0.01%,
cortisol < 0.02%, corticosterone < 0.001%.
Intraassay CV
6.6% (n=10) porcine plasma sample containing 5.8 ng/ml
6.8% (n=10) with 1.9 ng/ml.
Interassay CV
For an addition of 0.5; 3.0 and 6.0 ng testosterone / ml to an steroid free porcine plasma: 11.9, 8.8 and 15.8% (n=18), respectively.
Recovery (%) of different amount of testosterone added to porcine serum samples:


